Here is a term paper on ‘Water-Soluble Vitamins’. Find paragraphs, long and short term papers on ‘Water-Soluble Vitamins’ especially written for school and college students.
Term Paper on Water-Soluble Vitamins
Term Paper Contents:
- Term Paper on Thiamine
- Term Paper on Riboflavin
- Term Paper on Niacin
- Term Paper on Pyridoxine
- Term Paper on Pantothenic Acid
- Term Paper on Lipoic Acid (Vitamin N)
- Term Paper on Biotin
- Term Paper on Inositol
- Term Paper on Choline
- Term Paper on p-Aminobenzoic Acid
- Term Paper on Folic Acid
- Term Paper on Vitamin B12
- Term Paper on Carnitine (Vitamin BT)
- Term Paper on Ascorbic Acid (Vitamin C)
- Term Paper on Bioflavonoids (Vitamin P)
- Term Paper on Orotic Acid (Vitamin B13)
- Term Paper on S-Methylmethionine (Vitamin U)
- Term Paper on Pangamic Acid (Vitamin B15)
Term Paper on Water-Soluble Vitamins # 1. Thiamine:
ADVERTISEMENTS:
This is also known as vitamin B1, anti-beriberi factor, aneurin or antineuretic factor. Thiamine is a readily soluble, crystalline substance. It is insoluble in ether and chloroform and is slightly soluble in ethyl alcohol. In dry state, it is almost stable upto 100°C but is gradually destroyed in solution, or even after absorbing moisture. It is more stable in acidic solution. Alkaline pH has a destructive effect. During cooking, this vitamin is usually not destroyed till the temperature is raised above 100°C.
Thiamine possesses a. pyrimidine ring attached to a thiazole nucleus:
Intestinal micro-organisms can synthesize this vitamin whereas human ‘body is unable to do so.
Thiamine is found to be present in whole grains, legumes, nuts, yeast eggs, fishes, pork, beef, liver, heart, kidney and many other vegetables Milk and fresh fruits do also contain appreciable amounts of thiamine.
Functions:
Thiamine acts as a coenzyme in the carbohydrate metabolism. Thiamine in the form of thiamine pyrophosphate (TPP) acts as a cocarboxylase in the oxidative decarboxylation of pyruvic acid and α-ketoglutaric acid. It is suggested that insulin is required for formation of TPP from thiamine.
TPP is also required by the transketolases in pentose phosphate pathway of carbohydrate metabolism.
It has been reported that thiamine is necessary for normal uptake of oxygen by the brain tissue. In polyneuritic pigeons having severe deficiency of thiamine, uptake of oxygen by brain tissue is significantly lowered which is however increased by addition of thiamine.
ADVERTISEMENTS:
Deficiency Diseases:
In the deficiency of thiamine, growth has been found to be arrested in the experimental animals. In the birds, polyneuritis develops and they become unable to fly or even to stand. Rats develop bradycardia. Administration of thiamine exerts a curative effect in the birds as well as rats. It is suggested that in thiamine deficiency, level of pyruvic acid is raised as it cannot be oxidatively decarboxylated. Polyneuritis is believed to develop due to raised pyruvic acid levels.
In man, deficiency of thiamine causes a neurological disease, beriberi. In this disease, there occurs polyneuritis, muscular atropy, cardiovascular changes and edema. Other symptoms of the disease are feeling of fatigue, weakness, loss of appetite, headache, dizziness and insomnia. Beriberi in infants results when they are restricted only to milk diet. The symptoms include constipation, weakness, edema enlargement of heard cyanosis, and irregular pulse.
Thiamine deficiency occurs in alcoholics resulting into development of alcoholic polyneuritis. This is because of high calorific value of alcohol, thiamine, requirement is highly increased for oxidation of alcohol and if this is not met, it may lead to mild beriberi.
ADVERTISEMENTS:
Requirement:
Infants require about 0.3 to 0.5 mg thiamine. Children from one year to ten years need 0.7 to 1.2 mg thiamine. Males upto 50 years of age need 1.4 to 1.5 mg and females about 1.1 to 1.2 mg thiamine. Pregnant or lactating women require additional 0.3 mg thiamine in the diet.
Since, this is a water-soluble compound, hence it is readily excreted in urine. Thus when taken in excess, it is not stored like the fat-soluble vitamins. The question of hyper vitaminosis does not arise.
Term Paper on Water-Soluble Vitamins #
2. Riboflavin:
ADVERTISEMENTS:
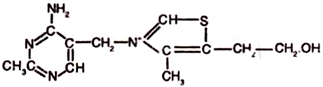
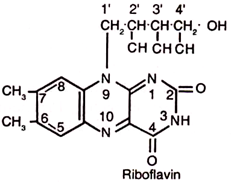
Riboflavin is also known as vitamin B2, or vitamin G. It is a water soluble, orange-yellow, crystalline compound. It is heat stable and acid-stable vitamin, however, it is decomposed on exposure to light.
Riboflavin is chemically 6, 7-dimethyl-9-D-1-ribitylisoalloxazine. It contains a sugar alcohol, ribitol, which is derived from ribose sugar. The attachment occurs between nitrogen at 9th position and carbon at 1’position.
Sources:
ADVERTISEMENTS:
Riboflavin exists widely in nature and the most important source is milk. Besides milk, the other important sources are liver, kidney fish, eggs, and leafy vegetables. The fruits and vegetable roots contain only moderate amounts of riboflavin. Other dietary sources, such as grains are relatively poor in riboflavin content. It is absorbed is the small intestine. It is not stored as such. It is mostly excreted through faeces and to a certain extent through urine.
Functions:
Riboflavin is involved in the metabolism of proteins, fats, carbohydrates and nucleic acids ‘by forming part of the flavoproteins. It forms the part of the molecular structure of flavin adenine dinucleotide (FAD) and flavin mononucleotide (FMN). The important enzymes such as L-amino acid oxidases, D-amino acid oxidases, succinic dehydrogenase, xanthine oxidase, cytochrome c-reductase all contain riboflavin in their molecular structure. The main function of this vitamin is concerned with oxidation reductions.
Deficiency Diseases:
Deficiency of this vitamin retards growth and there occurs inflammation of lips. Fissures develop at mouth corners. Skin becomes greasy and prone to infections. Eyes are also affected. There occurs inflammation of cornea, vision is blurred. Eyes appear to be red and dry. In pellagra, deficiency of riboflavin is usually observed.
Requirements:
Infants require about 0.4 to 0.6 mg riboflavin. Children upto 10 years of age require 0.8 to 1.2. mg riboflavin. In males, riboflavin requirement increases to 1.8 mg upto 22 years and thereafter, decreases gradually. In females, it rises upto 1.4 mg upto 22 years and then starts declining. Pregnant females require additional 0.3 mg, and lactating females require additional 0.5 mg riboflavin.
Term Paper on Water-Soluble Vitamins #
3. Niacin:
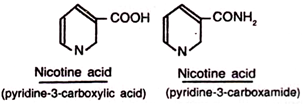
This vitamin is also known as nicotinic acid amide, or pellagra preventive factor (P-P factor), or vitamin B, Nicotinic acid also possesses vitamin activity.
Nicotinic acid contains a pyridine nucleus, possessing a carboxylic group at position third. In nicotinamide, the carboxyl group is present in the form of amide at position third of the pyridine ring. Nicotinic acid is a water-soluble, white, crystalline compound and is fairly stable when exposed to atmospheric air or heat.
Sources:
The rich sources of vitamin are liver, meat, fishes, eggs and vegetables. Whole wheat and unpolished rice do also contain vitamin in significant quantities.
Functions:
Nicotinamide forms the part of the molecular structure of nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP), and thus forms coenzyme of a large number of dehydrogenases.
The important known function of this vitamin is thus oxidative. It is involved in the oxidation of carbohydrates, lipids, deamination of amino acids, and metabolism of α-keto acids. It in the form of NAD, forms the normal component of electron transport chain.
Deficiency Diseases:
Deficiency of nicotinic acid has been shown to result in the development of pellagra in which patches develop on the skin; mouth and tongue develop soreness and inflammation. There occur achlorydna, diarrhoea, thickening and pigmentation of skin. Rashes appear in the body symmetrically.
In the advanced stages, there might occur nervous disorders. Deficiency symptoms of this vitamin are classified under three D’s, namely dermatitis, diarrhoea and dementia. Administration of nicotinamide relieves these symptoms.
In dogs, a condition of black tongue develops which is characterised by lesions in the mouth, and development of postules on the inner surface of lips and cheeks, and intense salivation. This can be cured by administration of nicotinic acid.
Requirements:
Normal adults require about 13-20 mg nicotinamide per day. During pregnancy, lactation and active muscular work, nicotinamide requirement is further increased by 2-4 mg. In children, the nicotinamide requirement is found to be 5-16 mg.
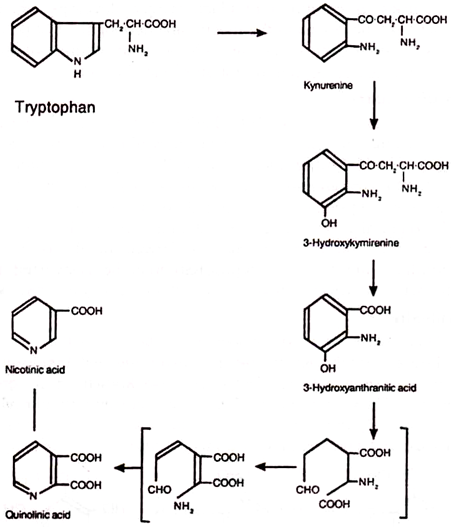
In old age, nicotinamide requirement is slightly reduced in comparison to adults. Even excess of this vitamin does not cause toxicity. In addition to dietary intake, nicotinic acid may be derived inn the body from tryptophan metabolism. Tryptophan may be converted into anthranilic acid which subsequently is changed into nicotinic acid.
Term Paper on Water-Soluble Vitamins #
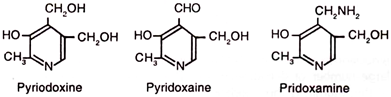
4. Pyridoxine:
There exist three compounds which belong to the group of pyridoxine, namely pyridoxine, pyridoxamine and pyridoxal, all showing vitamin activity. These are also known as B6 group vitamins. These are water soluble and sensitive to ultraviolet irradiation. Pyridoxine is resistant to heat but its derivatives pyridoxal and pyridoxamine are readily destroyed on heating.
The primary alcoholic group of pyridoxine is oxidized to aldehydic group in pyridoxal; and methylamino group in pyridoxamine. Pyridoxine, pyridoxal as well as pyridoxamine exist in the tissues as pyridoxine phosphate, pyridoxal phosphate and pyridoxamine phosphate.
Sources:
The rich sources of pyridoxine are egg yolk, meat, fishes, milk, whole grains cabbage and leguminous seeds.
Functions:
Pyridoxal phosphate is required as coenzyme in the decarboxylation reactions, brought about by various decarboxylases.
All the transaminases require pyridoxal phosphate. Pyridoxine is also considered to be essential for metabolism of unsaturated fatty acids. Vitamin B6 has been found to be associated with the active transport of amino acids into the cell.
The effect of B6 is antagonized by isonicotinic acid hydrazide (INH) which is used in the treatment of tuberculosis. Vitamin B6 has also been reported to promote transport of potassium across the membranes from exterior to interior.
Deficiency Symptoms:
Deficiency of this vitamin produces skin lesions. Prolonged deficiency leads to fall in hemoglobin content, mental depression and confusion. It may also cause vomiting diarrhoea, abdominal dystension and convulsions. Patients taking anti-tubercular drug. INH, mist some time develop peripheral neuritis. These abnormalities can however, be corrected by administering pyridoxine.
Requirement:
Normal adult man requires 1.6 to 2 mg pyridoxine per day. During second-half of pregnancy, pyridoxine requirement is increased to 2.5 mg per day Infants do also require about 03 to 0.4 mg pyridoxine per day. In patients receiving anti-tubercular treatment, the requirement of pyridoxine is increased to compensate pyridoxine deficiency created by INH.
Term Paper on Water-Soluble Vitamins #
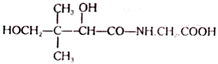
5. Pantothenic Acid:
Pantothenic acid is also known as vitamin B3. It is a water- soluble, yellow coloured compound, oily in existence. It is stable to heat, moisture, and to the action of oxidizing and reducing agents. However, it is destroyed when heated in alkaline or acidic solution. Pantothenic acid isolated from yeast has been found to have the following structure –
Sources:
The important sources of pantothenic acid are liver, kidney, eggs, lean-beef and milk. Among the plant sources are molasses, peas, cabbage, cauliflower, sweet-potatoes, yeast, potatoes and tomatoes.
Functions:
Pantothenic acid forms the part of the structure of coenzyme A and by virtue of that it participates in carbohydrate, lipid and protein metabolism.
In carbohydrate metabolism, it is involved in the activation of pyruvic acid (also obtained from proteins) to form acetyl CoA (active acetate) which can easily enter the Krebs cycle reactions and get oxidized; or can enter into biosynthetic pathway leading to the formation of long-chain and short-chain fatty acids, or steroids.
In lipid metabolism, pantothenic acid in the form of coenzyme A is involved in the activation of fatty acids forming their coenzyme A derivatives which can enter oxidative, or synthetic pathways. It is also involved in the biosynthesis of acetylcholine at the myoneural junctions. Coenzyme A is also involved in the disposal of ketone bodies.
In adrenal glands, cholesterol concentration is lowered in pantothenic acid deficiency. Coenzyme A has also been shown to be concerned with pigmentation of skin and hair.
Deficiency Symptoms:
In man, pantothenic acid deficiency is rather rarely created except under carefully controlled laboratory conditions. The deficiency can be created by feeding w-methylpantothenic acid (antagonist of pantothenic acid) 100 mg daily together with pantothenic acid deficient diets. Under such conditions, the vitamin deficient human subject develop irritability, restlessness, insomnia, fatigue, staggering gaits and gastrointestinal troubles.
Administration of pantothenic acid relieves the condition. In the past, it had been believed that deficiency of pantothenic acid was responsible for graying of hairs and this vitamin is an anti-graying factor. This view has now been disapproved on experimental grounds.
In rats, pantothenic acid deficiency causes retarded growth, lesser reproductive power, necrosis of adrenal-cortex and resorption of the litters. Cholesterol content of adrenals is lowered considerably. In chickens, pantothenic acid deficiency results into retarded growth loss of appetite and development of fatty liver. Deficiency of this vitamin may be created by feeding pantoyltaurine and ω-methylpantothenic acid.
Requirements:
The requirement of pantothenic acid in human beings has not been found out exactly. Usually 10 to 15 mg pantothenic acid is derived from the average diets per day.
Term Paper on Water-Soluble Vitamins #
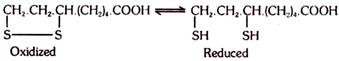
6. Lipoic Acid (Vitamin N):
Lipoic acid is also known as α-lipoic acid, pyruvate oxidation factor (POF) or protogen. It is a crystalline, insoluble substance and has been identified to be 6, 8-dimercapto-n-caprylic acid. Some authorities doubt its inclusion under vitamins.
Lipoic acid, is supplied to the organism with food. Yeast, meat products, and milk are especially rich in lipoic acid. The human requirement in lipoic acid is as yet unknown. For mammals, no dietary requirement has been conclusively recommended as yet.
Lipoic acid is involved in the oxidative decarboxylation of α-keto acids, such as, pyruvic acid and α-ketoglutaric acid. It acts in these reactions as an oxidising agent. It can be easily oxidised and reduced in the biological systems.
In medical practice, preparations of lipoic acid or its amide are used in the therapy of affected liver, diabetes mellitus, heavy metal poisoning, etc. The application of lipoic acid is commonly believed to favour the oxidation of carbon metabolism intermediates and improve the cell energetics.
Term Paper on Water-Soluble Vitamins #
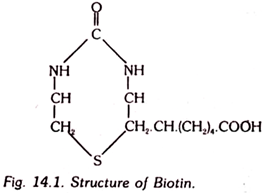
7. Biotin:
This vitamin, is also known as vitamin B7, coenzyme R or vitamin H. It is crystalline water-soluble vitamin having the following structure. A bound form of biotin, known as biocytin was isolated from yeast which was also found to exist in many animal and plant tissues. Chemically, this form has been identified to be N-biotinyllysine.
The important sources of biotin are liver, kidney, milk, molasses and to some extent other vegetables.
Functions:
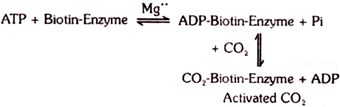
Biotin is involved in the carboxylation reactions. It is involved in the activation of CO2 in the following manner:
Later on, this CO2 can be fixed on other compounds such as pyruvic acid, propionic acid and acetyl coenzyme A. Active CO2 is also involved in the synthesis of carbonyl phosphate. Fatty acid synthesis is significantly reduced in biotin deficiency.
Biotin has been found to form prosthetic group of the enzyme propionic CoA carboxylase in pig heart which contains 4 moles of biotin per mole of protein.
Deficiency Symptoms:
Human beings require biotin in the diet. Deficiency of this vitamin can be created by giving low vitamin diet containing egg- white. Egg-white protein, avidin, combines with biotin and renders it unavailable to the individual. Sulfaguanidine can inhibit the formation of biotin by intestinal flora of micro-organisms and thus create its deficiency.
In human beings deficient in this vitamin, transient dermatitis is seen which is followed by anorexia, muscular pain and hyperesthesia. Administration of biotin relieves the condition. In rats, biotin deficiency leads to loss of hair and muscular control.
Requirements:
Although large amounts of this vitamin are synthesized by intestinal flora of microorganisms, yet 150 to 300 mg-biotin must be provided in the diet.
Term Paper on Water-Soluble Vitamins #
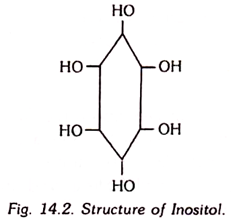
8. Inositol:
Inositol is also known as muscle sugar and has been identified to be hexahydroxycyclohexane. Myoinositol is a white, crystalline, water-soluble substance.
Animal tissues such as muscles, brain, red blood cells and eye contain inositol. It is also widely distributed in the plants e.g. fruits, vegetables, whole grains and yeast. Milk also contains inositol. Nine stereoisomers of inositol have been possible. Seven forms are optically inactive and two forms are optically active. Vitamin activity is found only in one optically inactive form, known as mesoinositol (myoninositel).
Inositol has been shown to exert a curative effect on fatty liver in the rats. In mice, its deficiency causes retardation in growth and alopecia.
In the human beings, its nutritional significance is not known definitely. Its lipotropic action is now generally agreed. In its presence, blood cholesterol level is not increased as expected on high cholesterol diets in the experimental animals. It has also been reported to act as anti-ketogenic compound.
Inositol is also present in the seminal fluid of human beings, rabbits and bull but its significance is not known. The requirement of this vitamin in the diet is not known, and even this is yet to be ascertained whether inositol is required in diet of human subjects at all.
Term Paper on Water-Soluble Vitamins #
9. Choline:
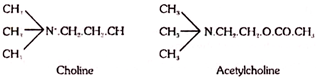
The inclusion of choline under vitamins is questioned by some authorities because some of the naturally occurring other compounds can substitute for choline. Choline is chemically trimethyl-hydroxyethyl-ammonium hydroxide and forms constituent of lecithin, and acetylcholine.
The important sources of choline are meat, egg-yolk, pancreatic tissue, bread, cereals and vegetables. It is fairly widely distributed.
Functions:
Choline is a well-known lipotropic factor. It prevents the development of fatty-liver as well as has a curative action. It also participates in transmethylation reactions. It forms part of acetylcholine and heos in myoneural transmission of impulses.
Deficiency Symptoms:
Deficiency of choline is accompanied by development, of fatty- liver which can be prevented by administration of choline. In puppies, growth is retarded and there occurs severe anorexia (loss of appetite). In rats, normal lactation; does not occur. In hens, laying of eggs is stopped Liver cirrhosis is often observed in the rats.
No definite deficiency symptom of choline alone has been shown in human subjects. Choline can be substituted by dietary methionine.
Term Paper on Water-Soluble Vitamins #
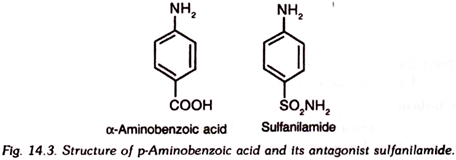
10. p-Aminobenzoic Acid:
p-Aminobenzoic acid (PABA) is a white, crystalline substance which is slightly soluble in cold water but fairly soluble in hot water.
It has the following structure:
PABA is found to be widely distributed. The rich sources are liver, yeast, rice-bran and whole wheat.
There have been observations that deficiency of PABA for sufficiently longer periods (in months), led to graying of hairs in human subjects and administration of PABA led to darkening of such hairs. It is however difficult to state whether this dietary factor is an essential requirement for darkening of hairs. Similar effect of PABA deficiency has been reported in the black-rats. In the rats, failure of lactation occurs in the deficiency of this vitamin.
PABA acts as an antagonist of sulfanilamide and some other members of sulfonamide group. These drugs show bacteriostatic property which is counteracted by administration of PABA. PABA thus promotes bacterial multiplication even in the presence of sulfonamide drugs.
PABA forms the structural part of another vitamin folic acid. Some authorities believe that PABA has a catalytic property only when it forms the structural part of folic acid. Some even question about inclusion of PABA under vitamins.
Term Paper on Water-Soluble Vitamins #
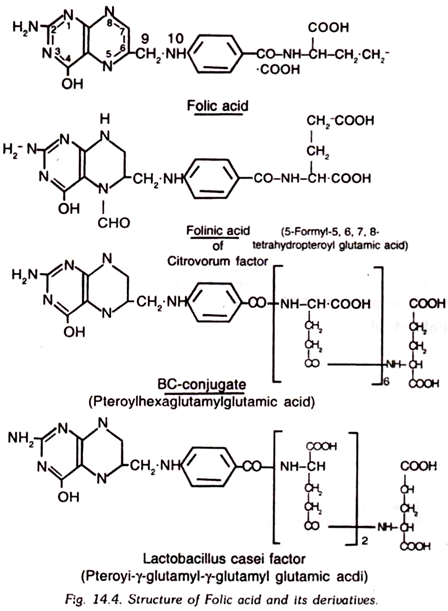
11. Folic Acid:
Folic acid is also known as pteroylglutamic acid (PGA). This vitamin is abundantly present in the green leaves and grass, and hence, this name folic acid (folium denotes leaf) has been given to it.
Folic acid is a yellow substance which is only slightly soluble in water. It is stable to heat in slightly alkaline medium, however, it is destroyed on heating in acidic medium. Sunlight destroys it. On storage at room temperature, its activity is gradually lost.
Folic acid is made up of glutamic acid, p-aminobenzoic acid (PABA) and pteridine. The portion of folic acid molecule which contains pteridine and p-aminobenzoic acid is known as pteroic acid.
Several other compounds which have been obtained from other sources having almost similar biological properties as folic acid are known. These include vitamin M, vitamin BC factor R, factor S, factor U, and Lactobacillus casein factor.
In folic acid group vitamins, the number of glutamic acid molecules differ and it may be one, three, or six. These compounds may be converted into PGA by the action of vitamin Bc conjugase which is found in most of the anal tissues.
Source:
This vitamin occurs widely in nature and is rich in the leafy plants. Besides leaves, the other important sources are cauliflowers, yeast, liver and kidney. Small amounts of this vitamin are also present in the tomatoes, banana, rice, corn, sweet-potatoes, pork and meat.
Functions:
Folic acid is very intimately involved in the metabolism of ‘one carbon moieties’ in various ways. In such reactions, folic acid is first reduced to tetrahydrofolic – acid (FH) in the presence of reduced NADP and ascorbic acid (vitamin C). The reaction is catalysed by folic acid reductase. Tetrahydrofolic acid can accept formate; formaldehyde (-CHO) or hydroxymethyl group (-CH2OH) forming 5-formyl- 5, 6, 7, 8 tetrahydrofolic acid or 5-hydroxymethyl- 5, 6, 7, 8-tetrahydrofolic acid.
These derivatives, later on act as donor of ‘one carbon moieties’ in various biotransformations, such as, in the conversion of glycinamide ribotide to formylglycinamide ribotide in purine biosynthesis, conversion of 4-amino-5-imidazole carboxamide ribotide into N-formyl-4- amino-5-imidazole carboxamide ribotide; conversion of d-UMP into 5-hydroxymethyl- dUMP and finally into TMP; glycine to serine and homocysteine to methionine.
Conversion of homocysteine to methionine requires N5-methyl-FH4, glycine into serine requires N5, N10 – hydroxymethyl- FH4, d-UMP to 5hydroxymethyl d-UMP ‘requires N10- hydroxymethyl-FH4, 4-amino-5 imidazole carboxamide ribotide to N-formyl-4-amino-5-imidazole carboxamide ribotide requires N10- formyl-FH4, and glycinamide ribotide to formylglycinamide ribotide requires N5, N10-anhydroformylfolic acid. Folic acid is needed for growth and blood formation.
Deficiency Symptoms:
Folic acid deficiency in man causes megaloblastic anaemia, glossitis, and disorder of gastrointestinal tract. In rats, deficiency of this vitamin leads to graying of hairs. In chicks, its deficiency results into anaemia. In monkeys, its deficiency results into diarrhoea, macrocytic anaemia, leukopenia, edema and lesions of mouth.
Folic acid deficiency might be created by use of folic acid antagonists, such as aminopterin (4-amino PGA) and amethopterin (4-amino-Niomethyl-PCA). Administration of aminopterin produces anaemia and leukopenta in guinea-pigs and rats. In children, administration of aminopterin has been recommended for lowering acute leukemia.
Requirements:
Infants upto one year of age require 50 µg folic acid: Children from one to three years 100 µg, four to six years 200 µg and seven to ten years 300 µg folic acid. Children above ten years, adults as well as old aged persons require 400 µg folic acid. Pregnant females require 800 µg and lactating females require 600 µg folic acid. In pathological condition e.g. macrocytic anaemia, its requirement is increased several hundred folds. It may range from 10-30 mg intravenously, or about 200 mg orally.
Term Paper on Water-Soluble Vitamins #
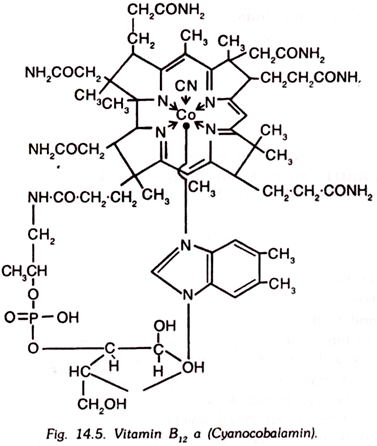
12. Vitamin B12:
Vitamin B12 is also known as antipernicious anaemia factor. It has been found to be identical with the extrinsic factor of Castle.
This is a deep red crystalline compound containing cobalt, cyanide and amino groups in its molecular structure and that is why, it is also known as cyan ocobalamin. It is soluble in water. It is stable to beat in neutral solution but is destroyed in dilute acidic or alkaline solutions. Its structure is very complex and has been worked out.
A molecule of vitamin B12 contains an atom of cobalt in the trivalent state. Three species of vitamins B12 are known, namely B12 a, B12 b and B12 c. In B12 a, cobalt is bound to cyanide, but in B12 b and B12 c, it is bound to hydroxyI group and nitrite group, respectively.
Vitamin B12 a is known as cyan ocobalamin. B12 b as aquacobalamin and B c as nitrito-cobalamin. Other compounds of this type are also known. Recent investigations upon human liver tissue have revealed that cyan ocobalamin forms only a very minor fraction out of the total cobalamins of the liver.
Three other major cobalamins isolated are adenylcobamide (AC), benzimidazelykobamide (BC), and 5, 6-dimetyl benzimidazoiylcobamide (DBC). Cyanide is not present in any of these three forms. DBC represents the main cobalamin of human liver. BC and DBC comprise 48-72 per cent of the total cobalamins in liver.
Sources:
The most important source of vitamins B12 is liver. Other but less important sources are milk, meat, eggs, and fishes. Vegetable foods generally lack these vitamins. Intestinal flora of microorganisms can also synthesize these vitamin.
Their absorption requires the presence, of an intrinsic factor which is found to be present in the normal gastric juice. B12 absorption is also increased in the presence of sorbitol, mannitol, sorbose and xylose.
Functions:
Vitamins B12 are essentially required for normal hematopoiesis (formation of blood) and erythrocyte maturation. In young animals, these show a growth promoting effect. Similar growth effect is observed in young children. These favour biosynthesis of nucleic acids. These are involved in the metabolism of glycine, serine, methionine, choline and methyl groups. These favour biosynthesis of thymine, choline, methionine and proteins.
Vitamins B12 are also involved in the enzymic conversion of methylmalonyl CoA to succinyl CoA. The exact role played by these vitamins in such reactions is yet to be investigated. Vitamins B12 are clinically used in cases of megaloblastic anaemia and to treat neurological disturbances. Vitamins B12 are also required for the formation of myelin sheath in the nerves; and for activation of -SH containing enzymes.
Deficiency Symptoms:
In the human subjects, B12 deficiency rarely occurs. Whenever deficiency occurs, it causes pernicious anaemia. This deficiency usually occurs due to lack of a low molecular weight mucoprotein in the gastric juice, known as intrinsic factor. In the lack of this factor, vitamins B (extrinsic factor) are not absorbed.
The intrinsic factor most probably helps in liberating B12 from natural protein-complexes and in their subsequent transport to blood. Intestinal juice is also known to secrete a factor which helps in the absorption of B12 in collaboration to intrinsic factor. Intrinsic facto, is also believed to help in the storage of B12 in the tissues, specially in liver. B12 deficiency also leads to demyelination of the nerves.
Requirements:
Infants upto one year require 0.2 µg B12– Children from 1-3 years require 1.0 µg; 4 to 6 years 1.0 µg and 7 to 10 years 1.0 µg B12. Children above 10 years, adults and old persons require 1.0 µg B12. Pregnant and lactating females need 1.5 µg B12 daily.
Term Paper on Water-Soluble Vitamins #
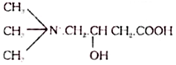
13. Carnitine (Vitamin BT):
Carnitine is a substance of wide occurrence, it is especially abundant in meat products. Carnitine is a true vitamin for insects (e.g. mealworm), but not for mammals in whose tissues it is synthetized from g-butyrobetain. The daily human requirement in carnitine is about 500 mg.
It is a derivative of betaine having the following structure:
Metabolism and Functions:
Carnitine biosynthesis is chiefly accomplished in the liver. The initial step is methylation of proteinic lysine producing ɛ-N-trimethyllysine. Then the lysine carbon chain is shortened by cleaving off the first and the second carbon atoms to form g-butyrobetain.
In the hepatic cell sap, the hydroxylation of g-butyrobetain with the participation of g-butyrobetain hydroxylase leads to carnitine according to the scheme:
To make this reaction possible, oxygen, 2-oxoglutarate, Fe2+, and ascorbic acid are needed.
L-Carnitine is the biologically active agent. Carnitine takes part in the transfer of fatty acid long-chained acyls and acetyl groups across the membrane lipid layer of mitochondria and, possibly, other organelles. For this reason, it exerts a pronounced influence on fatty acid oxidation and energy generation as well as on the utilization of mitochondrial acetyl residues in biochemical cytoplasmic reactions.
Other instances of carnitine activity may also be envisioned. There has been evidence that carnitine stimulates the exocrine, function of pancreas and exerts a favourable effect on spermatogenesis and mobility of spermatozoa. When carnitine is administered to animals, it enhances the energy generation in the respiratory chain of mitochondria of various organs, stimulates regenerative processes in the affected myocardium and in the spermatogenic epithelium of small seminiferous tubules.
There have been described cases of carnitine deficiency in humans associated with affected skeletal muscles. A distinct decrease in carnitine concentrations in the muscles have been observed, concomitant with muscular debility, dystrophy, and thinning of nerve fibres. Large doses of carnitine alleviate the course of this disease. Dietary deficiency of lysine depletes the supply of organism with carnitine.
In medical practice carnitine is a novel medicament, and in many instances its therapeutic effects await further elucidation. It is used for stimulating muscular activity, exocrine secretion of pancreas, and in treating myocardial dystrophic processes.
Term Paper on Water-Soluble Vitamins #
14. Ascorbic Acid (Vitamin C):
Fresh fruits and vegetables are the major source of vitamin C for humans – Especially abundant in vitamin C are wild-rose fruits. The daily requirement in vitamin C for the adult human is 50 to 100 mg.
Metabolism:
Absorption of ascorbic acid by simple diffusion occurs throughout the whole length of gastro-enteric tract, especially in the small intestine. In the blood, ascorbic acid is partly bound with proteins and partly occurs in a free state. When supplied to the tissues, ascorbic acid is retained therein to become bound with proteins.
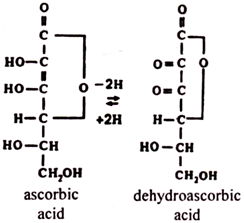
Free ascorbic acid can enter redox reactions. Most ascorbic acid (as estimated per tissue unit mass) is found in adrenal glands, liver, and lungs. The products of oxidative breakdown of ascorbic acid are dehydroascorbic, diketogulonic, oxalic, and some other acids. Free ascorbic acid and its catabolites are excreted in the urine.
Biochemical Functions:
Ascorbic acid acts as a hydrogen donor in enzymic redox reactions. It forms a redox pair with dehydroascorbic acid:
The reduction of dehydroascorbic acid to ascorbic acid in the tissues is carried out by ascorbate reductase with the participation of the reductant glutathione.
Ascorbic acid is involved in the following biological oxidation processes:
1. Hydroxylation of tryptophan to 5-hydroxytryptophan (in serotonine biosynthesis);
2. Conversion of 3, 4-dihydroxyphenylethylamine to noradrenalin;
3. Hydroxylation of p-hydroxyphenylpyruvate to homogentisic acid;
4. Hydroxylation of steroids during the biosynthesis of adrenocortical hormones from cholesterol;
5. Hydroxylation of β-butyrobetain in carnitine biosynthesis;
6. Intestinal reduction of Fe3+ ions to Fe2+ to provide for the bivalent iron uptake;
7. Release of iron from its binding with the transport protein transferin to facilitate the supply of iron to tissues;
8. Conversion of folic acid to its coenzymic forms;
9. Hydroxylation of proline and lysine residues in collagen synthesis.
It follows, therefore, that ascorbic acid is involved in the conversion of aromatic acids to produce certain mediators in corticosteroid synthesis, in hemopoiesis, and in the production of collagen which is a major extracellular component of connective tissue.
Ascorbic Acid Deficiency:
Deficiency in ascorbic acid results in the long-known disease called scurvy, or scorbutus. Acute forms of this disease manifest signs of disturbed biochemical functions of ascorbic acid. These show up in an impaired buildup of collagen and chondroitin sulphate of the connective tissue and its gradual destruction due to depolymerization and hydrolysis of the tissue’s fibrous structures.
This results in an increased permeability and fragility of the blood capillaries, which leads to subcutaneous hemorrhages. With vitamin C in short supply, the possibility of using the stored iron for the marrow cell hemoglobin synthesis and the participation of folic acid in hemopoietic cell proliferation is reduced. All these factors lead to the development of anaemia.
The evoked biochemical disturbances are concomitant with the outward scorbutic symptoms – the loosening and shedding of teeth (dedentition), hemorrhage from the gingivae, dolorous and endemic joints, pallor (anaemia) of the skin, hemorrhages, affected bones, impaired wound healing.
Practical Applications:
In medicinal practice, ascorbic acid is used in treating hypovitaminoses, in stimulating the hemopoiesis (alongside folic acid, vitamin B12, and iron), in strengthening the ruptured inner wall of blood capillaries, in stimulating the regenerative processes, and in medicating afflicted connective tissues and acutely diseased respiratory ducts.
Term Paper on Water-Soluble Vitamins #
15. Bioflavonoids (Vitamin P):
Fresh fruits and berries, especially black chokeberry, black currant, apple fruits, grapes, lemon as well as tea leaves and sweet briar fruits, are rich in P-vitamins. The dietary intake of these foodstuffs provides for the human requirement in biflavonoids whose recommended daily intake for the adult organism is 25 to 50 mg.
Chemical Nature of Bioflavonoids:
The active principles in bioflavonoids (P-vitaminic substances) are flavones structurally based on the flavone ring system:
Bioflavonoids constitute a very large group of naturally occurring compounds. In plants, some 2000 flavonoid and related compounds have been identified. Among these, anthoxanthins, anthocyanins, and catechols exhibit P-vitaminic properties.
Metabolism and Biochemical Functions:
Most flavonoids are mildly toxic. In the human organism, they are converted to phenolic acids. They are chiefly excreted in the urine in their pristine form or as phenolic acids and their conjugates with glucuronic and sulphuric acids.
In the tissues, the aromatic flavone ring system of bioflavonoids is presumably used in the buildup of biological materials, for example, ubiquinone; bioflavonoids may also play a specific role in the metabolism.
Many aspects of the bioflavonoid involvement in metabolism remain as yet unclear. The most marked feature of bioflavonoids is their ability to reduce the permeability and fragility of blood capillaries, i.e. to increase the capillary resistance.
P-vitaminic compounds interact with ascorbic acid in the control of connective tissue collagen formation and inhibit the depolymerization of hyaluronic acid as exerted by hyalouronidase. In doing so, flavonoids reduce the blood capillary permeability. Moreover, P-vitaminic compounds activate the tissue respiration.
Bioflavonoid Deficiency:
A deficiency of bioflavonoids in the organism is revealed by the symptoms of increased fragility and permeability of blood capillaries, punctate hemorrhages and odontorrhagia.
Practical Applications:
In medical practice, complex P-vitaminic preparations (sum total of tea leave catechols, sum total of black chokeberry flavonoids) as well as individual flavonoids (rutin, quercitol, hesperidin and their derivatives) are used. Of practical utility are also preparations combined with vitamin C – ascorutin, galascorbin, and tea catechols with ascorbic acid.
These preparations are recommended in the therapy of diseases associated with a damage and increased permeability of the capillary walls (capillarotoxicosis, allergic vasculitis, toxin poisoning, and radiation sickness) and reduced blood coagulability.
Term Paper on Water-Soluble Vitamins #
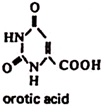
16. Orotic Acid (Vitamin B13):
Orotic acid is widely spread in animal foodstuffs.
Metabolism and Functions:
Orotic acid is a precursor in the biosynthesis of pyrimidine bases (uracil, thymine, and cytosine) and nucleotides. The biologically active form of orotate-orotidine 5′-monophosphate is involved in the synthesis of nucleotides and nucleic acids. Owing to this, orotic acid stimulates protein biosynthesis, cell division, growth and development of animals and plants.
No orotic acid deficiency occurs in humans; it may be presumed, however, that the growing organism or certain tissues in their regeneration periods may stand in increased need of this compound.
In medicinal practice, orotic acid is used as a growth factor for premature infants, for stimulating the hemopoiesis under certain anemias and for accelerating regenerative processes in afflicted tissues (for example, in the therapy of myocardial infarction and muscular dystrophy).
Term Paper on Water-Soluble Vitamins #
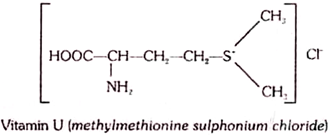
17. S-Methylmethionine (Vitamin U):
S-Methylmethionine is found in raw vegetables; it is especially abundant in cabbage. The active principle of cabbage juice exhibits the property to inhibit the development of experimental gastric ulcer; for this reason it has been named the antiulcer factor, or vitamin U (from the Latin ulcus, ulcer). Later, this compound was isolated in a crystalline form. By its chemical structure, it is a methylated derivative of methyonine
Vitamin U, similar to methionine, is an active donor of methyl groups; and thus, it facilitates the synthesis of choline, choline phosphatides, creatine, and other methylated compounds in the organism tissues.
Although the full picture of metabolic involvement of vitamin U is far from being clear, this species is profitably employed in the therapy of gastric ulcer, duodenal ulcer, and various gastritides. A lyotropic action of this vitamin is also possible.
Term Paper on Water-Soluble Vitamins #
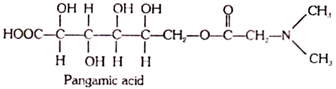
18. Pangamic Acid (Vitamin B15):
Pangamic acid occurs in many nutrients; however, its human requirement is unknown. The chemical structure of pangamic acid has not been elucidated unambiguously.
Presumably, it is an N- dimethylglycine ester of gluconic acid, although this compound is unstable and amenable to an easy hydrolysis:
Pangamic acid, similar to methionine, serves as a source of transferable methyl groups. It participates in the biosynthesis of methylated species (choline, choline phosphatides, creatine).
In medical practice, pangamic acid preparations are used as a lipotropic agent in the therapy of adipose infiltration of the liver, atherosclerosis, and certain other diseases; basic biochemical indications for the application of this drug are not always clear.