Here is a term paper on ‘Thyroid Gland’. Find paragraphs, long and short term papers on ‘Thyroid Gland’ especially written for school and college students.
Term Paper # 1. Introduction to Thyroid Gland:
The thyroid gland in the nongoitrous, normal adult weight 20-25 grams. The thyroid is enveloped by a thin capsule, which also serves to incompletely isolate Areas of the gland into lobules. The lateral lobules measure some 4 by 15 by 30 mm and the pyramidal lobe is often involved in disease of the gland.
The gland is very vascular some 5-liters of blood an hour flow through the normal adult thyroid. In comparison this amount of blood flows through the lungs about once a minute and through the kidneys in five minutes.
Histologically the thyroid consists of aggregates of follicles or acini which are lined by a single layer of epithelium the cells of which are generally medium cuboidal. The lumen of the follicle contains variable amounts of colloid material depending upon the functional, state of the gland.
ADVERTISEMENTS:
Since the epithelium has no basement membrane free-floating epithelial cells are often seen in the lumen of the gland. A great deal of work has been done in attempts to—associate. Golgi bodies vacuoles, mitochondria, and intracellular lipoid, material with the functional activity of the gland.
Some workers consider that the biosynthesis of the thyroid hormones occurs extracellularly in the colloid and that the follicular cells merely serve to collect and supply it to the lumen. There are many contradictory studies in this field, however, and other workers assign biosynthetic activity to various cell fractions.
As will be shown the follicular colloid represents the gland’s store of hormone. Because of this the thyroid is unique or it is the only endocrine gland to store appreciable amounts of hormone.
Thyroid gland activity is controlled at least in part by the thyrotropic hormone of the anterior pituitary. Stimulation by the pituitary hormone causes secretory alterations of the cytological components of the folicular cells hypertrophy and hyperplasia of the epithelium, vacuolization and resorption of colloid loss of hormonal iodine, and increase in vascularity of the thyroid gland. In turn, circulating levels of thyroxin appear to control the thyroid-stimulating, hormone of the anterior pituitary. This balm of control between two important secretions may be likened to a feedback mechanism.
ADVERTISEMENTS:
This feedback system is more complex than that of a simple two-component system. Purves cites nine components of the feedback system. In some animal, species, other hormones such as the cortical hormones seem also to influence the dynamic control of thyroid function.
It is well recognized that the central nervous system is involved in the control of thyroid function. The hypothalamic aspect of thyroid control is a major field of study. It appears that thyrotropic hormone production can be influenced by factors introduced by way of the hypophyseal portal vessels.
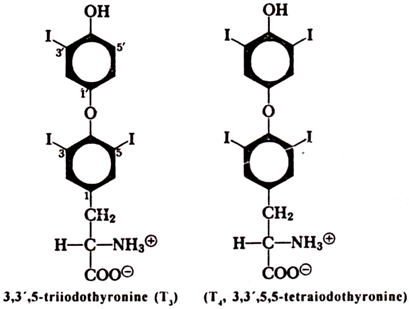
Term Paper # 2. Triiodothyronine and Thyroxine (T3 and T4):
The action of these hormones and the regulation of their production by the thyroid gland provide examples of many general principles, in addition to being important in their own right.
The thyroid regulates metabolic activity and promotes growth and development through the synthesis and release of thyroxine (tetraiodothyronine, T4) and triiodothyronine (T3) –
ADVERTISEMENTS:
Thyroxine is the major product of the gland, but triiodothyronine is more active and may be the only form bound to receptor proteins in the nucleus, thereby altering gene expression. We shall see that these hormones are made by iodinating specific tyrosine residues in a protein, thyroglobulin.
Specific effects of thyroid hormone include striking changes in gross appearance and activity. Lack of hormone can produce a listless, constipated, coarse-haired slow-pulsed individual who complains of being cold. Over-secretion of thyroxine or triiodothyronine leads to a garrulous, hyperkinetic individual with rapid heart rate, diarrhea, and huge appetite who complains of being hot. While these “classical” findings are easily discerned, the manner in which thyroid hormone produces these effects is not clear.
The increased basal metabolic rate led to a search for many years for uncoupling of oxidation from phosphorylation in mitochondria. Early claims of positive results were quietly abandoned, and we have no valid substitute. However, the absolute necessity of the hormone for normal fetal development, together with its specific binding by a nuclear protein, makes alterations in genetic expression the more likely possibility.
ADVERTISEMENTS:
The thyroid gland is a bilobed organ in the anterior portion of the neck. It is really a collection of individual glands in the form of follicles, which are circular in cross section with a central lumen in which the newly synthesized thyroid hormone still present in thyroglobulin is stored. Apical portions of the cuboidal cells comprising the follicles contain numerous microvilli and secretory granules on the luminal side.
Synthesis of Thyroxine:
Transport of Iodide:
The thyroid consumes about 70 to 100 µg of iodide per day for hormone synthesis, which it obtains by re-utilizing the iodide released upon degradation of the hormones, making up any deficit from the dietary intake. The daily intake of iodine in the United States typically ranges from 200 to 500 m.g a day. Dietary iodine is reduced to iodide and almost completely absorbed into the blood stream from the intestinal tract.
ADVERTISEMENTS:
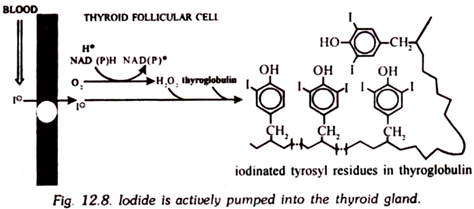
Iodide is actively transported into thyroidal cells through linkage to a (Na+ + K+)-ATPase system. A thyroid in a normal adult contains ca. 6,000 µg of iodide, whereas all of the rest of the body has only about 75 µg of inorganic iodide and 500 µg of organic iodide. Follicular cells are avid collectors of iodide from the blood stream, much more so than any other cells in the body. Indeed, hyperactive thyroid glands can be selectively and therapeutically destroyed by drinking the radioactive isotope, 131I, which is concentrated in the thyroid gland and destroys it by emitting gamma rays and electrons.
Thyroglobulin is almost completely confined to the thyroid gland, which synthesizes it to serve as a scaffold that holds some tyrosine residues in specific configurations for ready iodination and conversion to thyroxine and triiodothyronine. Other proteins can be iodinated, and indeed are to a small extent within the thyroid gland, but their tyrosine residues are not located at positions favourable for combination into the active hormones.
After synthesis, thyroglobulin is transferred to secretory vesicles and then released into the lumen. Carbohydrates are added during packaging for secretion, and this large protein (670,000 M.W.) contains some 280 carbohydrate residues. The polypeptide chain is rich in cysteine residues, with about 200, nearly all of which are in disulfide linkage.
Iodination of the tyrosyl residues in thyroglobulin is a complex process, not yet completely elucidated, which occurs in the apical portion of the cells – the part next to the lumen. The iodinating enzyme is a heme-containing peroxidase, which also travels through the cell as if it is to be secreted. However, it is probably retained in the plasma membranes or other structures at the lumen cell interface. (This interface has a complexly interdigitated morphology.)
ADVERTISEMENTS:
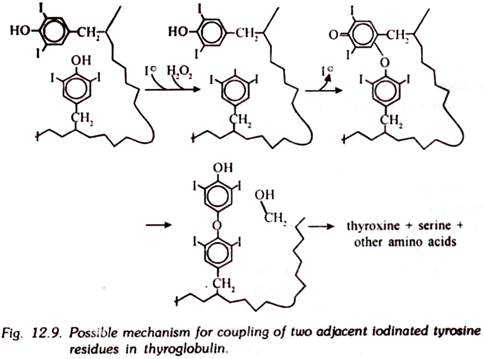
The sources of the necessary H2O2 also have not been defined; likely possibilities are the transfer of electrons from NADPH through cytochrome c to oxygen, or from NADH through cytochrome b5 to oxygen by extra mitochondrial enzymes. The mechanism of iodination may involve free radical forms of both iodine and the phenolate portion of tyrosine residues, which combine to form monoiodotyrosine residues. Further reaction forms diiodotyrosine residues.
The coupling of two molecules of diiodotyrosine to form thyroxine (tetraiodothyronine) may follow the scheme. Thyroglobulin is obviously constructed in a fashion that will facilitate iodination of residues that are in favourable positions for coupling to make the iodothyronines.
Triiodothyronine is generated by coupling monoiodo and diiodotyrosine in a similar fashion. Many proteins can be iodinated in vitro by the thyroid peroxidase system, but little thyroxine is formed. Human thyroglobulins from normal glands, on the other hand, had on the average only 15 tyrosine residues iodinated out of the 118 present per molecule, as analyzed by one laboratory.
Of these, roughly five residues were still present as monoiodotyrosine, and three as diiodotyrosine, but six had been converted to three residues of thyroxine and one had been converted to triiodothyronine (one residue in two molecules of thyroglobulin). The efficiency of formation of the iodothyronines increases as the iodine content increases in vivo, indicating that those residues are preferentially iodinated that are in appropriate positions for conjunction to form the iodothyronines, of which more than 80 per cent will be the tetraiodo compound (thyroxine) in individuals with an adequate iodine supply.
Secretion of the Iodothyronines:
Secretion is initiated by the return of iodinated thyroglobulin to the cell through fusion of droplets of the lumen contents with lysosomes to form phagosomes, in which the protein is hydrolyzed to its constituent amino acids. The released iodinated residues include both mono- and diiodotyrosine, as well as the coupled tri- and tetraiodothyronines.
Iodine is removed from the iodotyrosines and becomes available for re-utilization. The iodothyronines pass through the plasma membrane and basement membrane to enter the blood stream where they circulate almost entirely bound to protein.
Circulating thyroxine and triiodothyronine are bound almost quantitatively to three proteins – thyroxine-binding globulin, which is the most important carrier, thyroxine-binding prealbumin, and albumin, so the concentration of the free hormones is only 4 x 10-11 M for thyroxine, 1 x 10-11 for triiodothyronine. Even so, it is the free hormone concentration that is the important determinant of metabolic activity. The half-lives in the blood are ca. one week for thyroxine and one day for triiodothyronine.
Peripheral Metabolism of Thyroxine:
Only one third of the triiodothyronine in the periphery is secreted as such by the thyroid gland. The remainder arises from deiodination of thyroxine, primarily in the liver, kidney, and heart. Only 30 to 40 per cent of the thyroxine is converted to triiodothyronine, with the balance of 15 to 20 per cent converted to inactive tetraiodoacetic acid and other products. Some is excreted in the bile as glucuronides or ester sulfates. A significant amount is converted to reverse T3 (3, 3’5’ triiodothyronine) which has negligible metabolic activity.
Term Paper # 3. Control of Thyroid Gland Activity:
i. Regulation by the Adenohyphophysis:
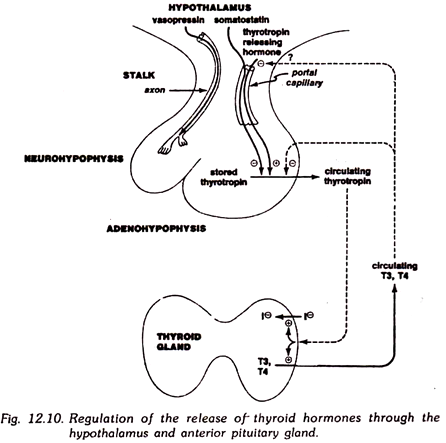
The secretion of thyroid hormones is under the control of another endocrine gland, the adenohypophysis or anterior pituitary gland. Some of the cells in the adenohypophysis secrete a polypeptide hormone, thyrotropin (thyroid stimulating hormone, TSH), which reaches the thyroid gland through the blood and stimulates it to release thyroxine and triiodothyronine.
The thyrotropin-forming cells of the anterior pituitary gland are in turn stimulated by another hormone, the thyrotropin releasing hormone, which is an oligopeptide formed in the hypothalamus and is transported to the anterior pituitary gland through a portal circulation in the pituitary stalk.
This sequence of cascade activations, hypothalamus to anterior pituitary gland to thyroid gland, is typical of a sequence affecting other endocrine glands, and therefore deserves more detailed attention. Like other cascade mechanisms, it greatly amplifies signals, with one nanogram of hypothalamic hormone causing the release of many times as much thyrotropin, which in turn stimulates the release of much more thyroxine from the thyroid gland.
The pituitary gland is a collection of differentiated cells that act as a message center. Signals reach it from the hypothalamus, cerebrospinal fluid, blood plasma, and nerve terminals. In response to these signals, the involved cells transmit their messages in the form of peptide hormones. Anatomically, the pituitary gland is enclosed in a bony box, the sella turcica, with a stalk connecting the gland to the hypothalamus.
It is really two distinct glands. The posterior pituitary gland, or neurohypophysis, secretes the hormones vasopressin and oxytocin, which reach the gland for storage in secretory vesicles through the axons of specialized nerves arising in the hypothalamus, where these hormones are made in the cell bodies.
The anterior pituitary gland synthesizes, as well as secretes, several polypeptide hormones. The controlling messages in the form of hypothalamic hormones reach it through a portal system of capillary vessels draining the median eminence of the hypothalamus and passing the blood through the anterior pituitary gland before it is returned to the heart.
All of the hormones of the anterior pituitary gland are polypeptides, and their secretion is under the control of other factors in addition to the hypothalamic hormones. Now let us consider the steps in this general scheme that directly affect the formation of thyroid hormones.
Thyrotropin releasing hormone is a tripeptide; it is almost certainly made by cleaving a larger precursor because it contains a pyroglutamyl group.
The cells synthesizing this hormone in the hypothalamus release it upon stimulation of alpha adrenergic receptors by noradrenaline; this, therefore, is an important locus of control over thyroid action through the nervous system. These include somatostatin, which has an inhibitory action on secretion of hormones by the anterior pituitary gland. Somatostatin is also made in the pancreatic islet cells and specialized cells of the upper gastrointestinal tract.
Thyrotropin is made by particular basophilic cells in the anterior pituitary gland known as thyrotrophs. (There is a curious admixture in the literature of the stems tropic, meaning turning, and trophic, meaning feeding, in connection with these hormones.)
These cells are stimulated to release thyrotropin upon the binding of thyrotropin releasing hormone to their plasma membranes. The mechanism is not clear-cut; as was mentioned in the general discussion, these peptide hormones may act by initiating several changes, including activation of adenyl cyclase, activation of protein kinases by other routes, and altering the permeability and release of Ca2+.
A major control of thyrotropin secretion is an inhibition by triiodothyronine or thyroxine. As the circulating iodothyronines increase in concentration, they shut off the release of thyrotropin, the signal for their own formation. This very sensitive feedback loop is the device by which the blood hormonal concentration is kept relatively constant; similar devices are used for regulating the concentrations of hormones produced by other glands under control of the anterior pituitary.
The action of the hypothalamic thyroid releasing hormone and other regulating factors may be regarded as devices for overriding the primary control through feedback inhibition. The secretion of thyrotropin is also inhibited by somatostatin from the hypothalamus.
Thyrotropin consists of an α and β subunit. The same polypeptide chain is used to make the α subunit of other hormones from the anterior pituitary (luteinizing hormone and follicle stimulating hormone), which resemble thyrotropin in being glycopeptides. A variable number of residues are removed from the end of the α chain in these hormones. Thyrotropin and the other hormones get their distinctive characters from their β subunits.
Within minutes of administering thyrotropin to experimental animals, the cells of the thyroid gland begin synthesis of mRNA, active transport of iodide into the cells, and reabsorption of thyroglobulin from the lumen. Again, these responses may be mediated in part by activation of adenyl cyclase and in part by other effects on the plasma membrane of the thyroid cells.
Thyrotropin has less well-defined functions in other tissues. Perhaps the clearest demonstration came from the discovery that the hormone could be partially hydrolyzed by pepsin to produce a large fragment containing most of the β chain, but only part of the α chain.
This fragment was devoid of activity on the thyroid gland, but it stimulated development of the retro-retinal tissues in the guinea pig to produce the exophthalmos (protruding eyeball) sometimes associated with hyperthyroidism. These tissues were being stimulated by thyrotropin, not by the iodothyronines.
ii. Regulation by Iodide Concentration:
Changes in the concentration of circulating iodide cause opposite changes in the release of the iodothyronines. Fart of the effect comes from a direct inhibition of the follicle cells by iodide; part can be indirect. Iodide has an inhibitory effect on the thyrotrophs in the anterior pituitary; as its concentration rises, less thyrotropin is released.
Clinical Disruption of Thyroid Hormone Production:
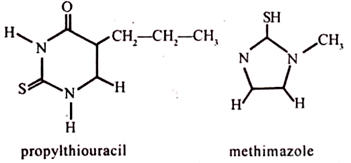
Administering radioactive 131I or plying cold steel are widely used and effective treatments for hyperactive thyroid glands. More sophisticated attacks upon the biochemical pathways involve blocking of specific sites with drugs. Monovalent anions (thiocyanates, perchlorates, and nitrates) inhibit active transport of iodide. Perchlorate can be used in humans.
Propylthiouracil and methimazole are clinically useful drugs that interfere with iodination of tyrosyl residues:
Propylthiouracil also interferes with the deiodination of thyroxine to triiodothyronine in target cells. Other drugs are useful in blocking the acute symptoms of excessive thyroid hormone. Propranolol, a beta adrenergic blocker, and reserpine, which depletes the supply of catecholamine, will relieve nervousness, fever, and hyperkinetic activity.